Acetone is a solvent with a broad range of uses. It’s widely recognised as nail polish remover, but it’s also used in cleaning, paint thinning, and even as a chemical reagent. There’s a lot of research underway on how acetone replacement can be done – it’s quite a challenge. But why go to all the effort?
Why replace acetone?
You can reduce risk in the workplace by removing acetone from use. Its flammability and high vapour pressure are a hazardous combination, creating a risk of fire and explosion. Storing large volumes on-site will make your insurers nervous and raise costs. For workers who are regularly exposed to acetone, it can cause dizziness, drowsiness, and headaches. Research suggests it may even affect menstrual cycle length. Finally, disposing of acetone can be expensive, as it’s a hazardous waste that needs special treatment.
Swapping acetone with a greener alternative can be better for your workers and your customers, but also better for your bottom line.
What makes a chemical ‘green’?
The definition of ‘green’ is complicated, and often depends on the application. Most simply, a greener chemical is better for humans and the environment. What ‘better’ means varies by use case, but can include:
- Less hazardous to human health
- Fewer harmful environmental emissions
- Less waste in manufacturing
- Improvements in process efficiency
- Biodegradation
- Easy reuse or recycling
- Biobased or renewable feedstock
You can read more about choosing green solvents here.
In some cases, green chemicals can let you enter new markets. Biobased products made from renewable sources and materials – algae, plant waste, even marginal crops – offer opportunities to stay ahead of your competitors, and sometimes even reduce costs. As long as product performance doesn’t slip, authentically green products can significantly increase market share.

Photo by Nick Fewings on Unsplash
What makes acetone unique?
Solvent Characteristics
Acetone is a solvent with some unique properties. It is ‘polar aprotic’ – it has a strong affinity for polar compounds such as ions, and doesn’t have hydrogen atoms (protons) capable of forming hydrogen bonds. This puts it in a category of solvents that are very good at dissolving plastics and adhesives. Yet, acetone also has non-polar features. It can dissolve organic compounds such as hydrocarbons, making it useful for thinning petroleum-based products. Its ability to mix with water increases its versatility.
To accurately compare acetone to other solvents, it’s useful to map its solvent properties. One helpful way of doing this is with Hansen solubility parameters (HSP), which describe a solvent’s performance based on three characteristics – dispersion, polarity, and hydrogen bonding ability. Putting each characteristic on its own axis creates a three-dimensional picture of solvent space. Solvents that cluster together in this space are likely to dissolve similar substances, so looking for solvents that are close together in the Hansen space can help identify potential replacements.
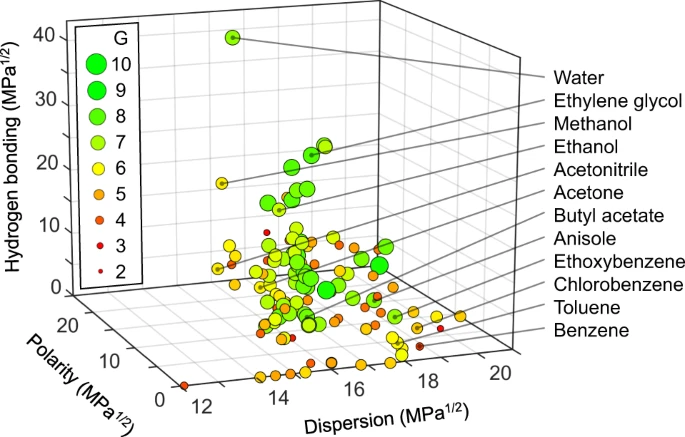
Solvents in Hansen space by Larsen et al. Image source.
Volatility
Acetone is a very volatile liquid, which means it quickly evaporates at room temperature and pressure. This can be both good and bad. It is useful for cleaning applications where fast evaporation without residue is desirable. But some types of cleaning require a long “dwell time” to lift the dirt or coating – in these cases, acetone doesn’t make the cut.
Volatility can also pose logistical challenges during solvent storage and use. Specialised, safe storage is needed to handle build-up of gas pressure, and speciality pumps may be needed for safe transfer. A volatile solvent is harder to recycle, as capturing the evaporated gas requires specialised equipment that is impractical in many applications. Recycling of the liquid alone will be inefficient, and regular input of solvent will be needed to replace what wasn’t captured, raising costs and reducing process sustainability.
Finally, volatile solvents are more likely to be released into the environment as a gas. This can cause issues with legislation around air pollution, as well as workers and consumers if the gas comes with a noticeable odour. As awareness grows of problems with indoor air quality, consumers increasingly prefer products that don’t pollute their homes.
What is acetone used for?
Acetone for nail polish removal
Nail polish removal is a large market for acetone. For basic and gel polishes, as well as acrylic nails, it is an effective solvent. It is widely used by home consumers as well as professional nail artists.

Photo by Valdas on Adobe Stock
However, nail salons often have poor ventilation. Employees are exposed to acetone in large quantities for eight or more hours per day, causing health effects. For customers, acetone can make nail beds brittle and cause damage to skin surrounding the nail. Combined with the potent chemical smell, these issues are driving adoption of acetone-free nail polish removers.
Cleaning with acetone
Acetone is widely used for cleaning and degreasing. Its volatility makes it well-suited for cracks and crevices since it can dry quickly, preventing damage caused by pooling solvent. A wide range of industries use acetone as a cleaning agent:
- Textiles – removing gums and oils from raw silk and wool
- Electronics – cleaning without damaging electrical components
- Household Cleaning – cleaning hard surfaces such as doors and windows, and sometimes furniture and fabrics
- Flooring – cleaning equipment after wood staining, cleaning paint spillage
- Adhesives removal – so large-scale it has its own industry sector
Acetone in paints and coatings
Because of its good polymer solubility and fast evaporation, acetone is a popular ingredient in formulation of paints and coatings. It can help dissolve other components of the formulation, lower viscosity, and improve evaporation of the final product. It is sometimes used to help remove paints and coatings, but is not the best solvent for this purpose.
Replacements for acetone
What makes a good acetone replacement?
The ideal acetone replacement has a lot of boxes to tick:
- Similar solvent properties
Should dissolve the same things as acetone - Non-toxic
Avoiding negative health effects - Not a volatile organic compound (VOC)
Industrial emissions of VOCs are restricted by a variety of laws around the world, making a non-VOC alternative highly desirable - Balanced volatility
Staying useful but reducing solvent loss via evaporation - Biobased
Made from renewable resources such as waste plant matter - Cost
Similar to or cheaper than acetone is ideal - Easily recyclable
Low volatility will ease recyclability, but other factors also affect it - Smell
Consumers will prefer a better-smelling replacement
Replacing acetone with similar solvents
In some industries, it’s common to replace acetone with a very similar solvent, like methyl ethyl ketone (MEK) or ethyl acetate. However, these similar solvents typically have similar hazards. They perform well, and can be helpful if the goal is to develop an “acetone-free” product – but this label can be misleading if the hazards are still there, and we don’t recommend this approach for building trust with consumers.
What acetone replacements are on the market?
Chemical manufacturers have designed some direct safer replacements for acetone:

Replacetone
This is a water-based mixture of potassium carbonate and surfactants, including nonylphenol ethoxylate (NPE). NPE is corrosive to the eyes, but as it’s used at a low 5-10% concentration in this product and is slow to evaporate, this is not a major hazard. Replacetone’s low volatility means it can’t replace acetone in applications where fast evaporation is required. When used in cleaning, Replacetone emulsifies resins rather than dissolving them. The resins sink to the bottom of the container, and the clean liquid on top can then be poured off and reused. It is biodegradable and has a mild smell.

Prosolve
Prosolve – formerly Surfasolve – is a mixture of plant esters, surfactants, and other non-hazardous ingredients, making it a very safe acetone replacement. Like Replacetone, it suspends resins in solution, then allows the solids to settle to the bottom so the cleaning liquid can be reused. It can also remove adhesives and degrease tools. Prosolve’s low volatility limits its applications, but from an environmental perspective, it is 100% biodegradable.

Bio-Solv
A carbon-neutral ethyl lactate blend made mostly from corn. Bio-Solv is potentially corrosive to eyes, and a skin and respiratory irritant. It has similar solvent properties to acetone, particularly in paint removal. Its flash point of 56 °C makes it less flammable than acetone. It is also less volatile than acetone, meaning it’s more recyclable but has different applications in cleaning. It’s reported to have an unpleasant smell, but is 100% biodegradable.

VertecBio ELSOL AR
This is a biobased mixture of ethanol, ethyl acetate, and some proprietary ingredient(s), so its hazards are similar to those of acetone. It’s potentially corrosive to eyes, and a skin and respiratory irritant. Its applications lie mostly in formulating paints, coatings, and inks. It’s highly flammable, with a flash point of 4 °C. It’s carbon neutral and 100% biodegradable.

AcraStrip 950
This is a mixture of bio-solvents and surfactants, targeted for resins, gel coatings, and adhesives. It’s an irritant to skin, eyes, and respiratory systems. It is non-flammable, but still volatile like acetone. It’s biodegradable, has a mild smell, and can be filtered for re-use, as grease, resin, and other contaminants will settle to the bottom of containers.
| Replacetone | Prosolve | Bio-Solv | VertecBio ELSOL AR | AcraStrip 950 | |
|---|---|---|---|---|---|
| Solvent properties (similarity to acetone) | Similar, but emulsifies resins | Similar, but emulsifies resins | Very similar | Similar: some good, some bad | Similar: claims better |
| Health hazards | Potentially corrosive to eyes 2% of mixture has unknown acute toxicity | No substances hazardous to health at concentration used | Potentially corrosive to eyes Non-toxic | Potentially corrosive to eyes Skin and respiratory irritant Non-toxic | Potentially corrosive to eyes Skin, eyes, and respiratory irritant |
| Physical hazards | Non-flammable | Non-flammable | Flammable | Highly flammable | Non-flammable |
| Environmental hazards | Low VOCs NPE is toxic to aquatic life | Low VOCs | Low VOCs | No VOC data | Very low VOCs |
| Volatility | Very low | Very low | Low | High | Very high |
| Bio-derived | No | Partially | Yes | Yes | Yes |
| Biodegradable | Yes | Yes | Yes | Yes | Yes |
| Recycling | Good | Excellent | Good | Excellent | Excellent |
| Smell | Mild | Poor | Very bad | No data | Mild |
Replacing acetone with different solvents
You should also consider other classes of solvent. One factory in the US has switched to propylene carbonate (PC) for cleaning fibreglass-reinforced polyester boats. PC is non-flammable, with a flash point over 100 °C – much safer than acetone. However, the really outstanding finding was that solvent consumption dropped by 90% after the switch. Workers also noted that the low volatility makes the solvent easier to use, as their tools can be left soaking for hours without fear of solvent evaporation creating resin clogs.
Acetone blends to improve performance
Mixing solvents together creates a blend that has different properties than its components. Blending acetone with another solvent can create a higher-performance product, harnessing some of the benefits of acetone and reducing drawbacks. It will also reduce acetone use, potentially improving health and safety or sustainability of your product. It’s not a perfect solution, but if nothing else works, a lower-acetone blend can be a decent alternative.
Non-solvent choices for acetone replacement
Depending on your goal, you could consider a non-solvent replacement for acetone:
Abrasion
You can remove paints and coatings by rubbing/scratching them off, using materials like steel wool, a nail file, or sand-blasting. Be careful though, it may damage the material behind the coating – particularly concerning for nail polish, where the ‘material’ is a person!
Freezing
Extreme cold (cryogenic cold, as in liquid nitrogen) can be an effective pre-treatment for paint removal. This is particularly effective with coatings on metal, where the paint will contract more than the metal. The detached paint can then be removed by abrasion. Liquid nitrogen is cheap, and since the atmosphere is already 78% N2, releasing nitrogen is environmentally safe. However, the harsh temperature can damage some materials (again, not appropriate for nail polish removal).
Which acetone replacement is best?
This is a complicated question with no singular answer. Your best option for acetone replacement could be any of the options mentioned, depending on what physical and hazard properties you’re looking for. You should consider and test a range of alternatives before committing to one (here’s how).
If all else fails, why not go slightly greener by finding a biobased acetone? Most acetone is derived from petrochemical sources, but biobased options are growing. Acetone-butanol-ethanol (ABE) fermentation uses bacteria to produce its namesake chemicals from waste biomass, and is making a comeback around the world. It’s the same chemical, but from a renewable source!
How to choose an acetone replacement
Some solvents, even those marketed to replace acetone, won’t dissolve the same things, resulting in poor product performance and wasted development time. This can be avoided with some computational modelling, using tools like Hansen solubility parameters (HSP) to predict solvent suitability for specific applications.
Target solutes, like specific coatings, have a solubility sphere which can identify solvents that are likely to dissolve them. Solvents outside the sphere are not likely to work, even if they are in the same solvent class as acetone (polar aprotic). It gets complicated!
If you’d like some guidance with using HSP, or want someone to identify the right replacement for your specific needs, Green Rose Chemistry can help – we love learning about new problems!
Further reading
If you’d like to read more on acetone replacement, here are some useful sources:
‘Safer Alternative Nail Polish Removers for Salons and Consumers’ by Katy Wolf
A thorough investigation of acetone alternatives for nail polish removal, including non-solvent options and solvent blends.
‘Investigating the Effective Removal of Gel Nail Polish with the Hansen Solubility Parameters’ by Abigail Giarrosso, University of Massachusetts Lowell
A Master’s thesis using an HSP approach to identify acetone alternatives. Giarrosso challenges the efficacy of artificial models for human nails commonly used in testing.
Maratek Environmental: ‘What is acetone and how is it used as an industrial solvent?’
A brief overview of acetone usage across different industry sectors.

