If you’ve ever worked in a chemistry lab, you’re probably used to taking solvents for granted—grab the right one off the shelf, run your reaction or analysis, and pour it in the flammable waste when you’re done. Recently, we’ve started to question whether we can use solvents more sustainably. Many common solvents are hazardous petrochemicals, creating a big environmental footprint problem as well as putting workers at risk. So what can we do? Are there green alternatives to hazardous solvents?
What is a solvent?
First, as always, we need some definitions. It might seem obvious what a solvent is – the liquid bit! – but the answer becomes less clear when using supercritical fluids, gaseous solvents, or reactive solvents that become part of the product.

A solvent can be defined most simply as one component of a solution, but the more familiar definition is a substance that is used to dissolve your target substance (solute). Solvents have found widespread use in most industries, playing key roles in manufacturing processes, coatings, adhesives, and cleaning products. They’re also critical in chemistry research, making reactions easier, more efficient, and often safer. Consequently, demand for solvents has grown to the order of 20 million metric tonnes per annum, and is worth tens of billions of US dollars to the global economy.
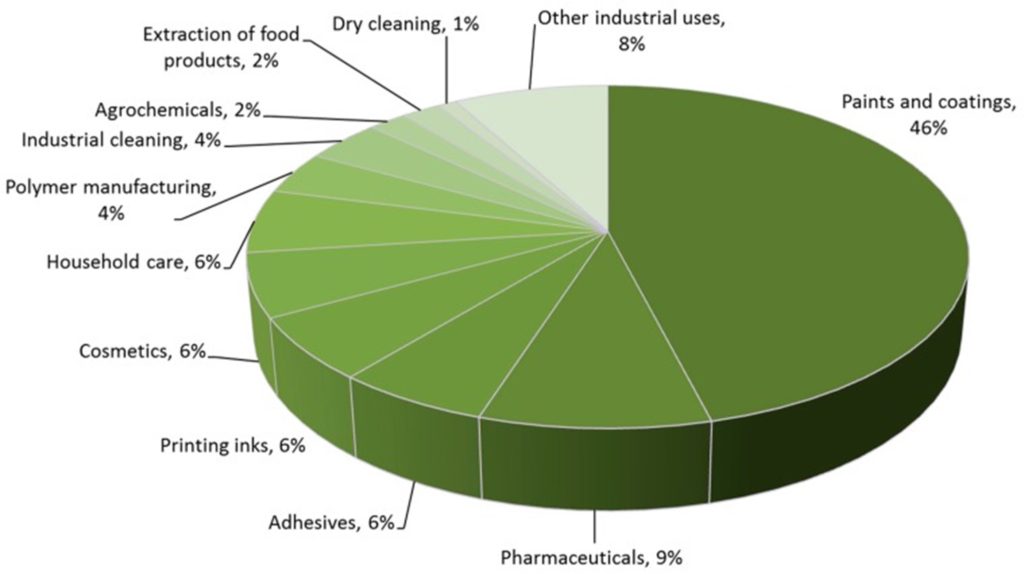
Solvent use by sector as reported by Clarke et al. Image source.
The widespread usage of solvents, and their tendency to be thrown away after one use, has made them an obvious target in improving the sustainability of the chemical industry. This is particularly important in the pharmaceutical industry, where research from GlaxoSmithKline (GSK) has shown that in the synthesis of a typical active pharmaceutical ingredient, solvent can account for as much 80–90% of the total waste by mass. Reducing solvent consumption or switching to green solvents can greatly improve process sustainability in almost any industry.
What is a green solvent?
The growing interest in environmental preservation and responsible use of resources, along with increasing pressure to reduce the use of harmful chemicals (see the United Nations Sustainable Development Goals, REACH, or the SIN List), is boosting demand for innovative solutions to the solvent problem. Green chemistry, a practice that seeks to design safer, more sustainable chemical products and processes, highlights safer solvents and auxiliaries as one of its 12 Principles of Green Chemistry. So, what exactly are green solvents and how can we go about finding sustainable alternatives to conventional solvents?
As always in sustainable science, pinning down an exact definition of “green” is challenging, and depends on the intended use. A solvent that evaporates slowly would be considered less green in pharmaceutical manufacturing, as more energy would be needed to remove the solvent from the target product. But in a fragrance diffuser formulation, slower evaporation can make the product last longer, reducing resource use as the product needs to be replaced less frequently.
For a solvent to be green, its negative impact on environmental and human health must be minimised. However, a full understanding of environmental impact requires an assessment of the solvent’s impact from manufacture to disposal, more commonly known as a life-cycle assessment (LCA). This sort of data is hard to come by, and often tailored to very specific applications.
In practice, it is more realistic to assess solvents on key properties for which data is available. These could be factors such as the hazard labels (flammability, aquatic toxicity, etc.), physical properties (such as boiling point, viscosity, density), the percentage of biobased feedstock, or cost. Finally, the performance of the solvent is critical to consider as part of the green definition—adopting a biobased solvent with a 30% lower carbon footprint is not sustainable if the substitution requires using 80% more solvent.
Choosing a green solvent
It’s become clear that selecting a green solvent that works for your product or process can be incredibly complicated. What practical steps can you take when considering a sustainable substitution?
Skip the solvent
The first option to consider is if an organic solvent (the conventional chemical option) is even needed in the first place. Companies in the paints, inks and adhesives industries have historically relied on large quantities of volatile organic compounds (VOCs) as solvents in their formulations. However, stricter legislation limiting the use of VOCs has led to a wealth of innovation in water-based formulations and solvent-free technologies, including powder coating and high-energy curing.
In chemical synthesis, mechanochemistry has emerged as a promising solvent-free approach. Instead of dissolving reagents in a solvent to mix them, solid materials are ground together with little or no liquid, and chemical transformations are facilitated through the input of mechanical energy. This technique is still in early research stages and has yet to see widespread adoption, although there is growing industrial interest, particularly in the preparation of metal-organic frameworks (MOFs) for applications such as gas-storage and CO2-capture.
Try a solvent selection guide
However, if solvents are crucial to your industry, there are a few tools you can use to help you pick an green solvent. One of the simplest approaches, which has attracted a lot of academic debate recently, is a solvent selection guide. These guides are developed by academic or industrial chemists who compare solvents across a range of properties and suggest possible greener alternatives for commonly used solvents.
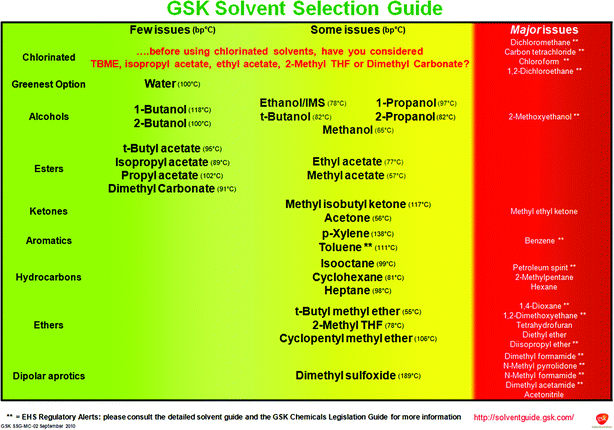
An example solvent selection guide from GSK. Image source.
There are a range of solvent selection guides to choose from, from companies such as Pfizer, Sanofi, and GSK, along with collaborative efforts from organisations like the ACS Green Chemistry Institute Pharmaceutical Round Table and CHEM21. For the most part, these guides are aimed at the pharmaceutical industry, but there are guides for some other industries like synthesis of perovskite solar cells, graphene processing, or leather manufacture. However, this approach should always be used with care, as regulations are always evolving and what works for one industry may not be appropriate for another!
Model multi-dimensional solvent properties
Another solvent selection approach is using software to help identify potential alternatives. While chemists are taught in school that “like dissolves like” and solvent selection is as easy as using a single polarity scale, the real science is significantly more complicated. Choosing a green replacement solvent based on plain polarity can be an expensive mistake.
Using more than one scale is a more accurate approach to predicting solvent behaviour. The most popular methods express solvent behaviour as three components, making them easy to compare in a three-dimensional space. Kamlet-Abboud-Taft (KAT) parameters, for example, measure three solvent characteristics in a lab and can predict how similarly two solvents will behave on this basis.
For those of you who don’t have a lab at hand, solvent characteristics that can be predicted from a computer are more practical. Hansen solubility parameters also use three characteristics, but are more desk-friendly. The software provides a predictive algorithm for solvent properties, and a database of experimental measurements for many polymers, allowing rapid prediction of polymer dissolution.
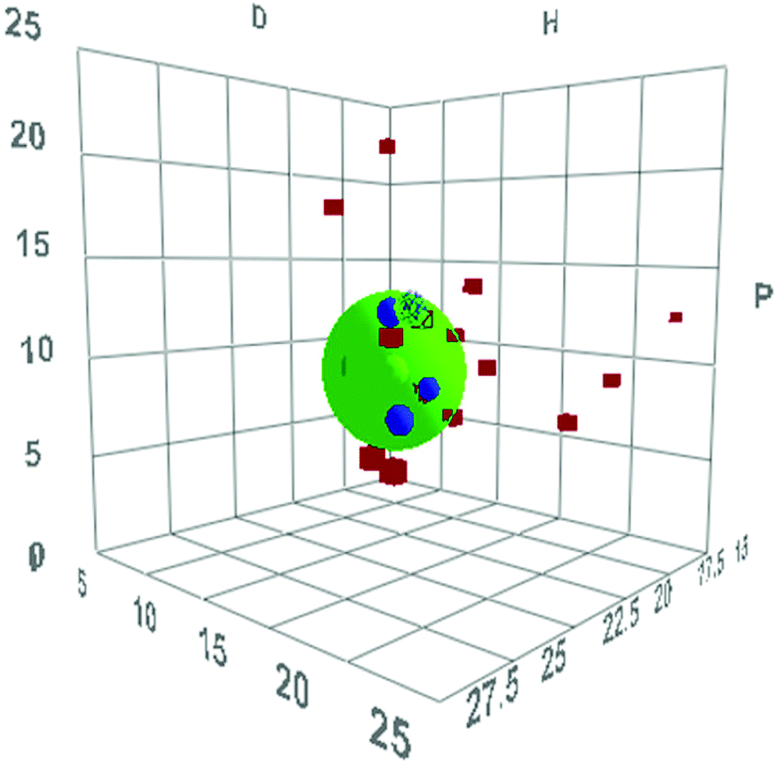
An example Hansen solubility plot for the swelling of poly(styrene) resins in organic solvents. Image source.
Some methods are more complex, using four or more characteristics (like the Abraham solute parameters) or quantum mechanical modelling to add a deep theoretical basis to the prediction (COSMO-RS). These tools are quickly becoming more advanced, and new tools involving machine learning are being developed to tackle difficult problems like polymer solubility.
It’s important to note that all of the tools above, like any tool, should be accompanied by a lot of research! There’s no easy or quick answer, and trying to predict solubility without fully understanding the theory can create costly mistakes.
Consider a weird solvent
Weird solvents, or “advanced reaction media” as scientists prefer to call them, are a quickly growing field. This category describes materials such as ionic liquids, deep eutectic solvents, supercritical fluids or switchable solvents, each of which has unique advantages and disadvantages.
Ionic liquids, which are generally salts with a melting point below 100 °C, are very slow-evaporating, solving one of the problems associated with conventional solvents. They are highly customisable, creating potential for use in niche applications that require very specific solvent properties. However, they can be expensive, sometimes toxic, rarely biobased or biodegradable, and are not yet widely available at industrial scale. The somewhat related deep eutectic solvents can solve some of these issues, providing options that are cheaper, less toxic, and sometimes biodegradable.
Supercritical fluids are used above their critical temperature and pressure, creating a state where the gas and liquid coexist. Supercritical carbon dioxide can be used as a non-toxic, non-flammable, low-cost solvent that works well for certain applications, like decaffeination of tea and coffee. The equipment required for supercritical solvents is very different to equipment used with conventional solvents, meaning switching over to a supercritical process is very capital-intensive.
Lastly, switchable solvents, which can change their physical properties in response to a stimulus, have been shown to be particularly useful where drastic changes in solvent hydrophobicity or ionic strength are needed, such as in separations. However, the potential eco-toxicity of the requisite materials has been highlighted as a concern.
Examples of green solvents
The research funding and focus that has recently been devoted to green solvents is beginning to generate results. One success story is Cyrene, which is becoming well-known as a safer, bio-based alternative to toxic dipolar aprotic solvents such as NMP and DMF. Cyrene has been demonstrated as a suitable alternative at lab scale for a range of applications including graphene processing, fabrication of polymer membranes and a number of chemical syntheses.
BASF has demonstrated the potential for industrial use of ionic liquids in their BASIL process, scavenging hydrochloric acid in the synthesis of a phosphine photoinitiator. This process is the first to use these novel solvents on a multi-ton scale.
Lastly, the Pure-CutTM technology was recently recognised by the US Environmental Protection Agency for green chemistry innovation in using supercritical carbon dioxide as a lubricating fluid during metal fabrication. This approach sidesteps the capital cost of supercritical systems, serving as a drop-in replacement with existing machine tools. It also utilises little to no oil, reducing hazard and toxicity while performing as well or better than conventional options.
So…can solvents be green?
As always, there is no simple answer for every situation. There are green solvent alternatives out there for every industry, but in some cases the options are not ideal – for example, alternatives exist for industrial cleaning solvents, but no good replacement has yet been found for hazardous halogenated solvents in vapour degreasing of sensitive equipment. In other cases, a green solvent can actually improve product performance or enable entry into a new market, creating significant value.
There are now a huge range of options to consider when replacing a petrochemical solvent, ranging from biobased versions of the same solvent to alternative reaction media to eliminating the solvent entirely. Research is ongoing, and more innovative solutions are being developed now, making it challenging to stay current and make the best choice. Computational tools and selection guides can help, but must be used carefully, and can result in mistakes if they’re not fully understood.
How Green Rose can help
If you don’t have the time to become a green solvent expert, Green Rose Chemistry can help – that’s what we do! We work with all industries and would love to hear about your specific solvent issue or question. Reach out today for a free consultation.

