Isopropyl alcohol (also called isopropanol or IPA) is a common solvent with a wide range of uses in industry and consumer products. It is used extensively as an antiseptic (rubbing alcohol) most notably in hand sanitiser. A large portion of it is also used in the production of acetone. However, there is a particular interest in replacing IPA in printing inks – so why and how?
Why replace IPA?
IPA is a very high production solvent found commonly in a variety of consumer products and used widely in industry. So why consider it for replacement?
Almost all IPA comes from the hydration of propene from petroleum distillation, which makes it undesirable as we transition to a biobased economy. It is a highly flammable liquid with a flash point of 12°C and a vapour pressure of 6.02 kPa at 25°C. In terms of physical hazard, it’s not quite as dangerous as acetone. But in a poorly ventilated space, IPA can emit narcotic fumes that pose a risk to workers. It is also a serious eye irritant, which may mean greater PPE requirements in the workplace.
In addition to safety and sustainability concerns, there are economic reasons to move away from IPA. It’s a very popular disinfectant, making it susceptible to supply issues when a spike in demand occurs. This happened during the first wave of the COVID-19 pandemic, when there was a dramatic increase in demand for hand sanitiser. The price of IPA rose dramatically, causing budgetary issues for printing operations that rely on it. Although it is a low cost solvent, it evaporates quickly so may need to be replaced frequently in applications such as component cleaning. This can ultimately lead to higher operating costs.
Swapping isopropanol with a greener alternative can be better for your workers and also better for your bottom line.
What makes a chemical ‘green’?
The definition of ‘green’ is complicated, and often depends on the application. Most simply, a greener chemical is better for humans and the environment. What ‘better’ means varies by use case, but can include:
- Less hazardous to human health
- Fewer harmful environmental emissions
- Less waste in manufacturing
- Improvements in process efficiency
- Biodegradation
- Easy reuse or recycling
- Biobased or renewable feedstock
You can read more about choosing green solvents here.
In some cases, green chemicals can let you enter new markets. Biobased products made from renewable sources and materials – algae, plant waste, even marginal crops – offer opportunities to stay ahead of your competitors, and sometimes even reduce costs. As long as product performance doesn’t slip, authentically green products can significantly increase market share.
What makes IPA unique?
Solvent Characteristics
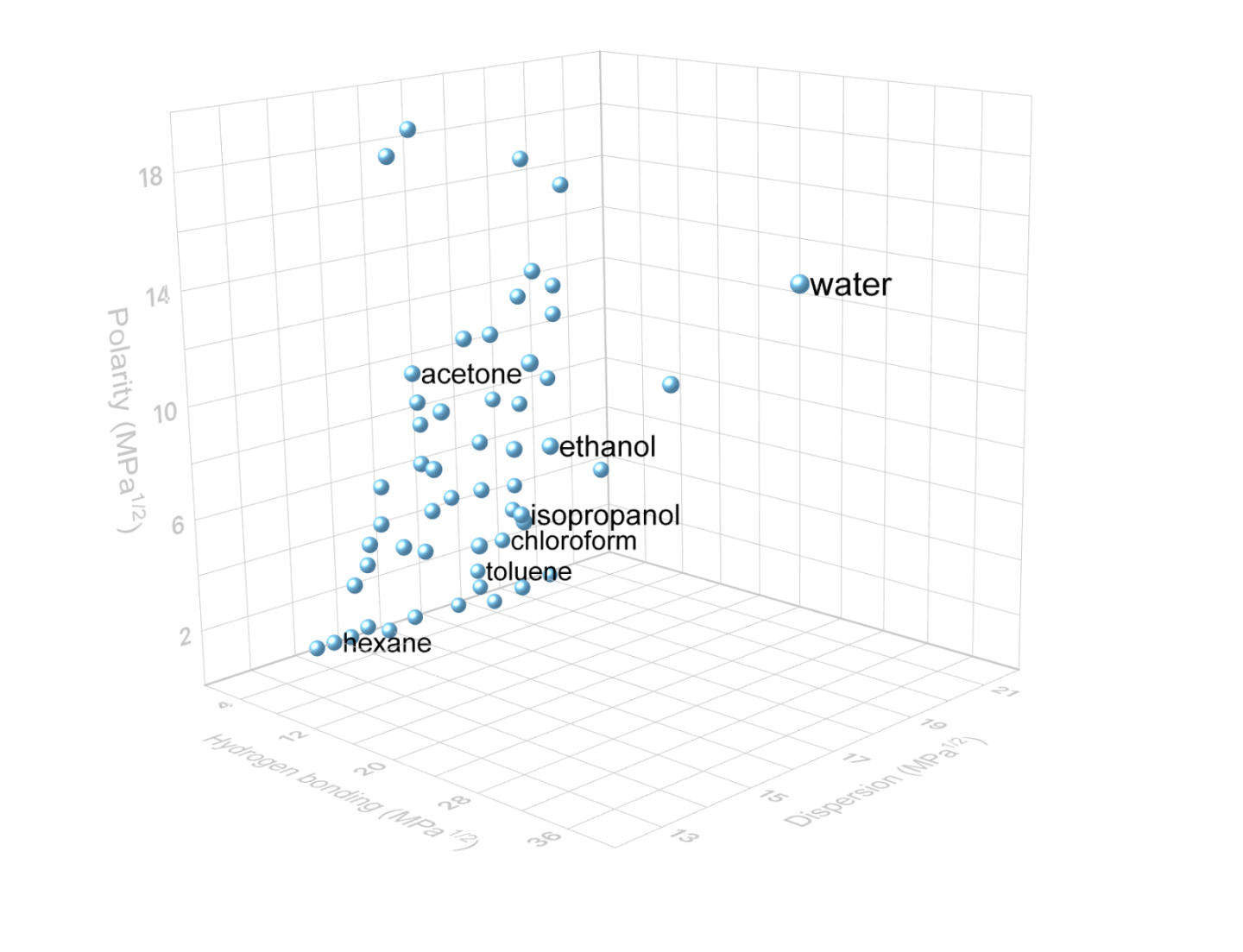
IPA is the simplest secondary alcohol. It is a polar protic solvent with a mid-range polarity that has some ability to dissolve both polar compounds, such as ions, and nonpolar compounds, such as grease residues. This means it is effective as a cleaning agent and general solvent, while its antimicrobial properties allow widespread use as an antiseptic (rubbing alcohol).