Earth’s supply of helium is running low. It’s the 2nd most abundant element in the universe, but it’s mostly found in stars – not so much on planets. Since we currently use a lot of it, we need to find alternatives, saving helium for when it’s really needed. If we’re going to run out of it, is helium environmentally friendly?
Why is helium in short supply?
Helium is really light. So much so that Earth’s gravity can’t hold it – any helium released into the atmosphere floats off into space and is lost to us forever. However, that’s not the only problem.
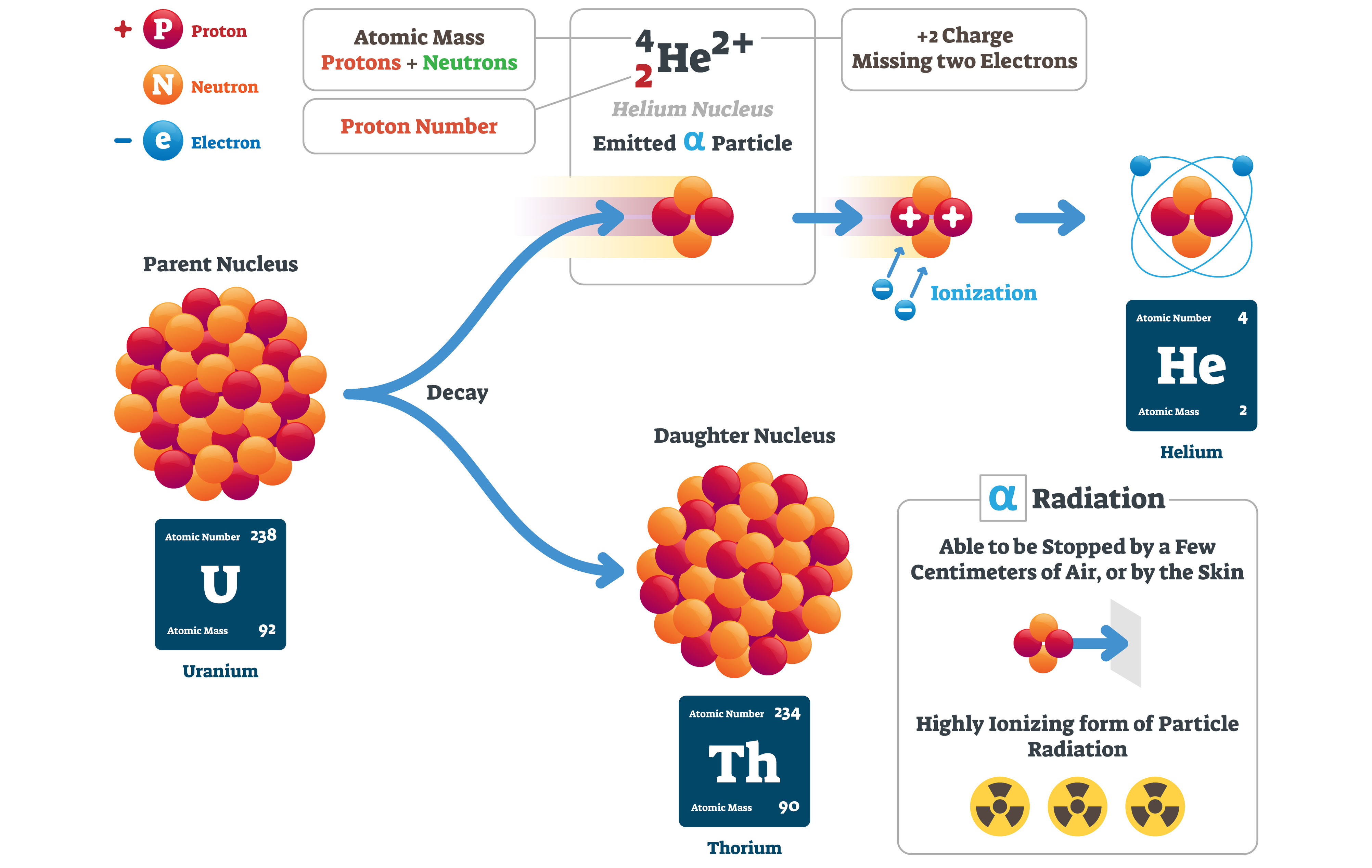
Helium can’t be synthesised. It’s an element – you can’t combine chemicals to produce it. The helium we have on Earth is a product of radioactive decay. An unstable uranium atom undergoes a process called alpha decay, ejecting an alpha particle – two protons, two neutrons, but no electrons, so not a full atom. If this happens in a sealed area, such as an underground pocket of space, the alpha particle is trapped, and can gather electrons over time, eventually becoming a stable helium atom.
Alpha decay of uranium, producing a helium nucleus.
The helium gas from radioactive decay collects in underground reservoirs, combining with other, more common gases. This means our main source of helium is natural gas deposits, found deep underground. These are mostly full of fossil fuel gases like methane, with a tiny amount of helium (only about 0.3% by volume).
We can’t speed up alpha radioactive decay. So, helium is under the same constraints as fossil fuels – it’s not renewable in our lifespans. It’s estimated that Earth’s helium reserves accumulated over 4.7 billion years, and will be many millions before a reasonable amount has been produced again. It’s a scarce, non-renewable resource.
The USA’s National Helium Reserve (NHR) is a critical source of helium for the world, and it is rapidly depleting. In 2012, the USA was responsible for about 78% of the world’s helium supply, 30% of which came from the NHR. Demand for helium far exceeds the supply, and this is only going to get worse with time. Estimates from 2019 suggest that the NHR has 80-100 years of helium supply remaining.
Why do we need helium?
Helium has many desirable properties. It’s completely inert – it takes absurdly high pressures and temperatures to make it react with anything. It also has the lowest boiling point of any material known, making it indispensable for superconducting materials.
It’s not a greenhouse gas, and is non-toxic. In terms of health and safety, it’s an excellent material. The only problem is the limited supply.
Cryogenic cooling
The use of helium as a cryogenic (e.g. very cold) cooling agent has enabled amazing advances in science and medicine with magnetic fields. In chemistry research, a technique called NMR (nuclear magnetic resonance) spectroscopy allows highly accurate identification of chemicals, speeding up drug discovery and other R&D processes involving new chemistry.
In medicine, MRI (magnetic resonance imaging) is a diagnostic imaging technique based on the same physical principles, but applied to bodies rather than chemicals. It lets doctors see inside a patient’s body without having to cut them open. MRI is really good for imaging tissues (the fleshy bits) and is excellent for locating tumours, among other things.