Silicon is an element found all around us. It accounts for ~28% of the earth’s crust by mass and has made its way into fruits, vegetables, beer and our buildings. It plays an important role in human physiology, making up our skin, hair, connective tissues, and bones. So, whilst it’s not going to make your beer healthy, it is essential for human health. We have found a cornucopia of uses for silicon in daily life. This includes being a semiconductor in electronics, a constituent of bricks, and in something called silicone.
What is silicone?
Silicone is largely recognised as a sometimes colourful, smooth, rubbery material, finding uses in children’s toys, kitchenware, and most controversially, breast implants. With recent movements to cut down on plastic, people looking for greener and more sustainable alternatives sometimes turn to silicone. But is silicone really a green material? What makes it different from plastic? Much like silicone itself, the answers aren’t so black and white.
![]()
Silicon vs. silicone
First, let’s clear up some confusion around the difference between silicon and silicone. Silicon refers to the naturally-occurring chemical element (Si), which is a hard, brittle material that looks like metal. Silicone is an artificial material that contains silicon (Si), oxygen (O), and other elements, forming a long polymer chain. Comparing silicon to silicone is like comparing graphite to a plastic bottle—while the same chemical elements are involved, the materials are completely different.
![]() Silicone is actually a chemical misnomer as it was originally thought that silicone polymers were silicon-based ketones. We now know that the chemical structure is different, but the name stuck and the word has been widely adopted. If you’re speaking to materials scientists, you may hear the more accurate name ‘polysiloxane’ (derived from a contraction of polymer, silicon, oxygen, and alkane). In this post, we’ll be using the common ‘silicone’ to mean long-chain polymers, and ‘siloxane’ for shorter chains with similar chemistry (oligomers).
Silicone is actually a chemical misnomer as it was originally thought that silicone polymers were silicon-based ketones. We now know that the chemical structure is different, but the name stuck and the word has been widely adopted. If you’re speaking to materials scientists, you may hear the more accurate name ‘polysiloxane’ (derived from a contraction of polymer, silicon, oxygen, and alkane). In this post, we’ll be using the common ‘silicone’ to mean long-chain polymers, and ‘siloxane’ for shorter chains with similar chemistry (oligomers).
How silicone is made
Silicone is made through some intense industrial chemistry. First, siloxanes are produced at massive scale by combining elemental silicon with chlorine using expensive fluidised bed reactors The siloxanes serve as building blocks for a range of silicone polymers, including everything from silicone rubber to liquid cosmetic additives. The properties of silicone can be tailored for specific applications by changing the chemistry of the siloxanes. Most siloxanes produced are methyl siloxanes. Methyl siloxanes can have a variety of structures and are categorised as either cyclic or linear siloxanes. The market is currently dominated by polydimethylsiloxanes (PDMS), which accounts for 80% of total siloxane compounds and is approved as a food additive for its antifoaming properties (E 900).
What makes silicone unique?
The Si-O bond has high energy, which means it is sturdy and takes significant energy to break. It also has a partial ionic character (sharp difference in electronegativity between Si and O), which is responsible for many of its properties. These properties make silicones uniquely useful for industrial applications, some of which have been listed in the table below.
| Silicone properties | Uses of silicone | How silicone improves products |
|---|---|---|
| ● Water, salt, and corrosion resistant ● Low surface tension ● Thermally stable ● Flexible ● Mostly lower toxicity | ● Construction ● Lubricants ● Electrical devices ● Food and drink ● Healthcare ● Cosmetics ● Textiles | ● More aesthetically pleasing ● Easier to use ● Longer lasting ● More stable ● Smoother |
When silicones were invented, their effects on the environment and human health were not considered fully. There is no known natural product that contains a silicon-carbon bond, and the natural environment has no way to handle this chemistry—silicones do not biodegrade. Most of the silicone we create will at some point enter the environment. As such, the responsibility falls upon us to understand and deal with the problem we’ve created.
Are silicone and plastic different?
Let’s be clear – silicones aren’t the eco-friendly alternative to plastic some claim they are. They still require fossil fuels, aren’t biodegradable, and the majority eventually ends up in landfills. The main difference is that silicones have a silicon and oxygen backbone with hydrocarbon side groups. Plastics have a carbon and hydrogen backbone. The silicon in silicone is abundant, but the carbon is usually sourced from fossil fuel feedstocks.
When compared to most plastics, silicone lasts much longer due to its stronger bonds, so it needs to be replaced less. This is because the Si-O bond (443.5 kJ/mol) found in silicones is higher energy than a C-C bond (355.2 kJ/mol) found in plastics, which means it needs more energy to break. However, the recycling rates for both are poor, as neither material is highly recyclable. Both plastic and silicone have issues with leaching chemicals into food, but silicone seems less likely to.
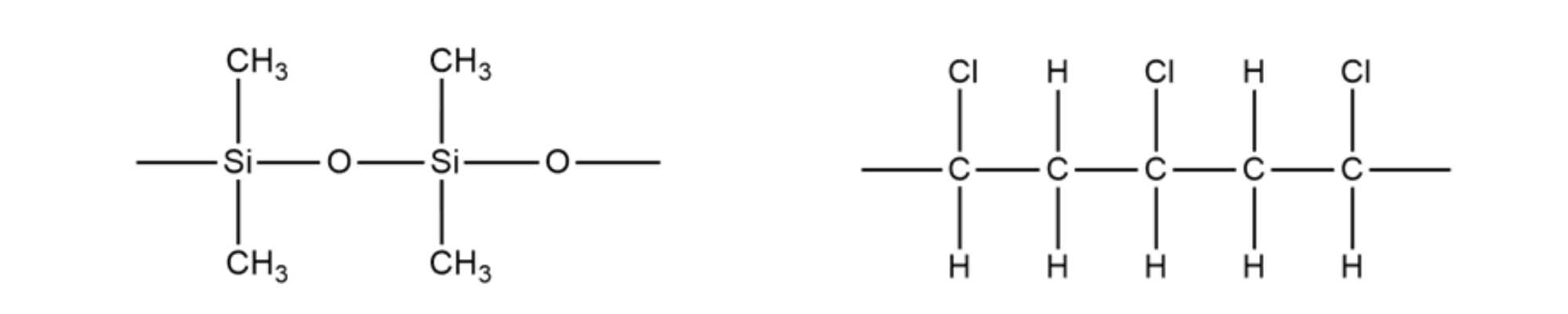
. Structure of PDMS Structure of PVC
Like plastic, silicone isn’t created in nature, so nature hasn’t evolved the tools to use it. So, what happens when silicones and their oligomers make their way into our environment?
D4, D5, and D6 exposure
Publications of the past touted PDMS as non-toxic because they are large molecules and insoluble in water. This view has changed in recent years. We now understand the potential threat associated with the smaller oligomers involved in the production and degradation of PDMS, as well as those directly added to consumer products.
We can’t really talk about the safety of silicones as a group, it’s too vast. We must instead refer to the safety of particular compounds. Here, the oligomers D4, D5, and D6 are briefly evaluated as they have recently drawn a lot of attention in the media.

Structure of D4 Structure of D5
Relatively recently, we became aware of the potential of siloxanes to be environmental pollutants. Once the methods were developed to detect these compounds it was found that D4 and D5 are ubiquitous. They are what’s known as cyclic volatile methyl siloxanes (cVMS) and the numbers 4, 5, and 6 represent how many siloxane groups each substance contains. They are intermediate materials in the production of PDMS’s, so their widespread production has brought a lot of D4-D6 into the environment via waste-gas, wastewater, and solid waste. The seriousness of this issue became obvious when in 2011, Genualdi et al. published their analysis of Arctic air. They found D4 and D5 concentrations of up to 44.3 ng m−3, which showed the alarming extent of siloxane migration. Siloxanes released so widely into the environment would surely find their way into humans and animals. What would happen in this eventuality?
Scientists have been investigating the various routes of exposure such as air, food, and skin. They concluded that we are exposed to siloxanes by the environment (water bodies, sewage, soil, air, and dust) as well as cosmetic and personal care products. Several studies have reported on the toxicity associated with siloxanes in laboratory animals. Some have reported that they are carcinogenic and toxic to the nervous, reproductive, and immune systems of animals. A lower molecular weight is regarded as more dangerous to humans, so D4 is considered more dangerous than D6. This is because low molecular weight compounds are more likely to overcome cell barriers. More recent research is focusing on the ability of siloxanes to overcome the barrier of the skin.
Food safety
Silicone rubber is a material that we regularly use with food, so it’s important that we know the safety data. In one study, the silicone bakeware was found to have D4-D6 oligomers (polymer fragments). After 6 hours of contact between infant formula and a silicone baking sheet at 40 ºC, no siloxanes were detectable in the formula. However, when an ethanol/ water mixture was used, small amounts of D4-D6 did migrate, but clearly, this doesn’t represent a typical use case. A 2019 study using silicone baking moulds did not contaminate cake at levels considered hazardous to health. However, they recommended moulds should be precleaned by heating and warned that consumers should follow the temperature suggestions closely.
A 2021 study warns of so-called ‘badly tempered materials’ that are marketed with volatile organic compounds 2.53% higher than is recommended. They found that these silicone rubber baking moulds released 18 different types of silicone oligomers to the food simulants they used. The full extent of the health risk of these compounds is not understood and neither is their impact on the odour and taste of food. They also note that there does not seem to be a good way for authorities or consumers to check whether the unreacted side-products have been properly eliminated by the manufacturer. This suggests that a label like ‘food-grade’ is not actually a useful indicator of its food safety.
Regulation
The findings above sent a clear message to regulators that this was an area requiring attention. In late 2016, the European Chemicals Agency (ECHA) restricted the use of D4 and D5 in leave-on cosmetics. In 2018, ECHA responded to warnings from the UK health and safety watchdog by classifying D4, D5, and D6 as substances of very high concern (SVHCs). This was mainly due to fears that siloxanes were accumulating and persisting in the environment. D4 was identified as a persistent, bioaccumulative, and toxic (PBT) and a very bioaccumulative (vPvB) substance. D5 and D6 were identified as vPvB substances. D4, D5, and D6 have been placed under REACH and were limited to a concentration of 0.1% in wash-off cosmetic products since February 2020 in the EU.
Not everyone agrees. The Global Silicones Council and four silicone manufacturers are taking legal action to challenge the decision by ECHA. They argue the decision is based on laboratory data, and the methods used were developed for carbon-based compounds, so aren’t appropriate for silicon-based compounds.
As of yet, there have been no restrictions placed on linear siloxanes, though they potentially behave similarly to cyclic siloxanes. More studies into the matter should be conducted to reach a decision.
GHG emissions
Silicone products seem to enable a reduction of greenhouse gas (GHG) emissions in a variety of areas. A study commissioned by the Global Silicones Council investigated 26 case studies for GHG emissions and found only two case studies with higher emissions than the product they were substituting. Furthermore, they found that GHG emissions generated by silicone products were outweighed by reductions by a factor of 9. This means that for every ton of CO2 emitted in their production, 9 tons of CO2 are saved by their use. Below are a few of the case studies they investigated.
| Case study | Benefits of using silicone |
|---|---|
| Silicone sealant | Do not become brittle so their insulating properties remain more constant |
| Silicone-based defoamers | Higher washer throughput makes pulp plant more efficient, less water must be vaporized, fewer process chemicals are lost |
| Green tyres | Less rolling resistance leads to fuel saving |
| Marine coatings | Prevent fouling of ship body which leads to fuel saving |
| Silicone rubber in motor construction | Allows motors to run at a higher temperature leading to more efficient motors which save fuel |
However, these results should be taken with a grain of salt. This is the only study of its kind, but GHG emission studies are notoriously hard to measure accurately. Furthermore, it must be questioned whether the Global Silicones Council has an interest in showing silicones in a favourable light due to their representation of major silicone manufacturers, despite being not-for-profit.
Recycling
The global capacity of silicone production is currently more than 8,000,000 tons. To overcome the issues related to the amount of CO2 required to produce silicone, capable recycling systems could be utilised to save natural resources and contribute to a sustainable society. Recycling is no easy task, and this is especially the case for silicone. Silicone is what’s known as a thermoset, so it doesn’t melt like thermoplastics, which causes a number of difficulties when trying to recycle or reuse them. The depolymerisation of silicones is currently our best method.
Here, silicone rubbers are depolymerised into monomers and then repolymerised. Depolymerisation reactions typically require high temperature, high pressure, long reaction times, and produce acid by-products with low yields. Silicone resins are even more difficult to recycle due to higher cross-link density. Recycling of silicone is not part of consumer recycling programmes. There are some companies that are willing to recycle it, but this is generally for commercial use.
Conclusion
We have explored a number of ways in which silicone can be evaluated for greenness. This is not the whole story because covering this vast topic in a single post would be impossible. Instead, it is our hope that this article serves as a basis for understanding the general position of silicone on the scale of greenness. Whilst not as damaging as single-use plastic, silicone is clearly not a green product, as it has a number of flaws. Low recyclability, high persistence, and dubious safety. The most important takeaway is that our understanding of these materials is patchwork and the gaps in our knowledge become even more apparent with time. We can safely say that we now have some knowledge of where our research efforts should be placed. The ongoing developments in this fascinating area of study could fill these gaps, so it’s crucial that silicones remain an object of research due to their widespread use in the modern world.

